Modify adult heart allocation 2016 2nd round
View commentsProposal Overview
Status: Implemented
Sponsoring Committee: Heart Transplantation
Strategic Goal: Provide equity in access to transplants
Effective dates
- Phase 1: 9/18/2018
- Phase 2: 10/18/2018
Adult heart allocation toolkit
Policy notice 1/2017 (PDF - 880 K)
Monitoring heart allocation policy changes (PDF - 2.0 M; 4/2019)
Policy notice 8/2017 (PDF - 365 KB)
Policy notice 7/2018 (PDF - 391 KB)
Board briefing 6/2018 (PDF - 758 K)
Board briefing 12/2016 (PDF - 1.5 MB)
UNOS and OPTN member impact summary 12/2016 (PDF - 861 K)
This proposal was originally sponsored by the Thoracic Transplantation Committee, which was dissolved July 1, 2020. The Heart Transplantation Committee will provide ongoing review and evaluation of this policy.
Executive Summary
The Thoracic Organ Transplantation Committee (the Committee) proposes modifications to the adult heart allocation system to better stratify the most medically urgent heart transplant candidates, reflect the increased use of mechanical circulatory support devices (MCSD) and prevalence of MCSD complications, and address geographic disparities in access to donors among heart transplant candidates. In response to significant comments received during the first round of public comment, and based on additional feedback and consensus-building after public comment, the Committee proposes the following modifications to the original proposal:
- Refining and tightening the qualifying criteria for candidates supported by veno-arterial extracorporeal membrane oxygenation (VA ECMO), percutaneous circulatory support devices, intra-aortic balloon pumps (IABP), and multiple inotropes to require evidence that these candidates are supported by these therapies for treatment for cardiogenic shock, rather than qualifying based on the presence of the therapy alone
- Criteria for determining presence of cardiogenic shock are based on American Heart Association definitions or the presence of end-organ dysfunction
- Placing additional restrictions on the duration for candidates may remain in statuses 1 through 3
- Candidates supported by the therapies above, which are intended for short-term, acute therapy for cardiogenic shock, will be limited to 14 days in the respective status unless the candidate exhibits contraindications to use of a durable device and has failed a weaning attempt
- Clarifying which mechanical circulatory support devices qualify a candidate for certain statuses, including limiting status 1 to candidates supported for biventricular failure with surgically-implanted, non-endovascular devices
- Requiring regional review boards to review cases external to their region
- Limiting the proposed broader geographic sharing scheme for the most urgent candidates to donation service area and Zone A (instead of through Zone B)
- Modifying the pediatric donor allocation sequence to limit potential negative impacts of the new adult heart allocation system on pediatric candidates
- Explicitly specifying the additional proposed data collection for the development of a heart allocation score in the future
Public comment: January and August, 2016
Read the August 2016 proposal. (PDF - 1.1 MB)
A heart allocation policy webinar was held on August 18th. You can find a recording at UNOS Connect, UNOS' learning management system.
Feedback requested
- Are the proposed indicators of cardiogenic shock appropriate?
- Should regional review boards review cases from other regions instead of their own regions?
- Should the current policy for sensitized candidates (permitting the transplant programs and OPO in the donation service area to agree to allocate a donor heart to a sensitized candidate even if the candidate is not first on the match run) remain in place in light of broader sharing?
- Which data elements on the list of potential heart allocation score data are likely to be incorporated into a heart allocation score due to their potential to predict waiting list mortality or post-transplant survival? Are there additional data elements that should be collected which the Committee did not include? Are there extraneous data elements on the list? Are there any data elements that should only be collected on VAD patients?
UNOS and OPTN member impact summary
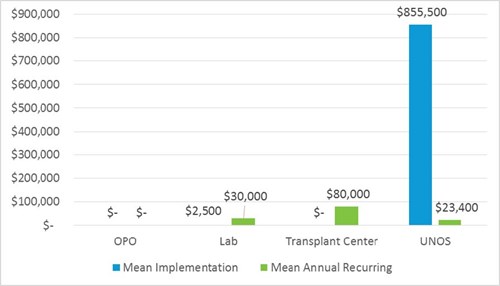
Project size/complexity
UNOS
Implementation is an enterprise-wide effort, requiring 11,040 hours in IT programming. Changes to the Waitlist and DonorNet systems, as well as UNOS staff systems are necessary. This is an entire overhaul of an allocation system similar to the revised kidney allocation system.
Substantial effort is also required of Policy (220 hours), Research (100 hours) and Instructional Innovations (100 hours) to assist with system changes, data collection, and member education on new policy.
Ongoing annual effort includes 293 IT hours.
Project size = Enterprise
Member
Lab
Implementation timeframe for all labs may be a few weeks. Staff training on the new allocation system and updating internal policies account for implementation. No additional staff are required to enact or maintain the changes.
Implementation costs can include staff training. Number of staff and salary will vary by lab. While unlikely, increased volume in crossmatch and testing may require purchase of additional instrumentation to support the increase.
Most labs should be able to absorb additional testing volume without additional equipment purchase, since similar tests are already performed for other organ types. If not, the cost for a Luminex analyzer to test for antibodies is approximately $50,000, with a $5,000 annual service fee.
Since the proposal simply requires that CPRA data be reported, and does not mandate extra testing, it is unlikely that increased recurring cost due to testing will be realized at all. In the case of increased testing, recurring costs account for additional antibody testing and crossmatching supplies for highly sensitized heart patients and any potential staff overtime hours to complete the additional work volume. The volume of additional virtual and physical crossmatches can be dependent on the size of the transplant centers served by the lab, accounting for a wide potential recurring cost range ($1,000 - $60,000). Costs can include purchase of additional reagents for testing, crossmatch supplies, and staff hours in testing and analysis.
Lab Methodology Notes:
- Labs assume running crossmatches and tests for an additional 2-8 sensitized patients (or an active list of 25 (small heart program) to 60 patients (larger heart program) per year. It is assumed about 10% of wait-listed patients might be considered “sensitized.”
- Unless volume is substantial, most labs should be able to absorb any additional costs.
Transplant Center
Implementation timeframe for transplant centers is estimated at three months to train staff and update internal policies. The costs are minimal.
Recurring costs are substantial. Broader sharing increases the number and distance/time of additional heart fly outs. A range of additional fly outs is estimated at 3 additional hearts for smaller centers to 11 at larger centers, potentially costing $30,000-$110,000 annually. Longer case time results in additional OPO staff time billed to Transplant centers. OPOs coordinate cases and arrange logistics, passing costs to transplant centers. Transplant centers receive reimbursement from insurance, but insurance reimbursement may not equal the full cost of the fly out. Additional transplant center staff time to record data for additional transplant volume may be required.
Transplant center methodology notes
- An average flight is estimated at $10,000
- Hospital cost per hour for heart transplant case is estimated at $750
- 20% of heart transplants are estimated to require flights
OPO
Implementation and recurring costs are minimal. If additional heart transplants and/or heart fly outs increase, additional staff time may be needed to coordinate. OPOs without call centers may be more burdened by additional staff time, but the minimal increase can likely be absorbed. Increased cost of heart fly outs and increases in medical supply purchases are passed to transplant centers.
Policy/Bylaws affected
Policies
- 3.7.B (Required Expedited Modifications of Waiting Time),
- 6.1 (Status Assignments and Update Requirements),
- 6.1.A (Adult Heart Status 1A Requirements),
- 6.1.B (Adult Heart Status 1B Requirements),
- 6.1.C (Adult Heart Status 2 Requirements),
- 6.2 (Status Updates),
- 6.3 (Adult and Pediatric Status Exceptions),
- 6.3.A (RRB and Committee Review of Exceptions),
- 6.3.B (Exceptions to Allocation for Sensitized Candidates),
- 6.4 (Waiting Time),
- 6.5.C (Sorting Within Each Classification),
- 6.5.D (Allocation of Hearts from Donors at Least 18 years Old),
- 6.5.E (Allocation of Hearts from Donors Less Than 18 Years Old),
- 6.5.F (Allocation of Heart-Lungs),
Bylaws
- Appendix K.5 (Transition Plan during Long-term Inactivity, Termination, or Withdrawal), and
- Appendix M (Definitions)
Summary of changes
- Statuses:
- Transition from a three status system to a six status system
- Broader sharing:
- Broader sharing for the most urgent statuses
What members need to do
Implementation of this proposal will have the largest impact on transplant programs. You will be required to submit more data than is currently required for each of your candidates during each status change and at defined time intervals. You will need to add the required data for all your heart candidates registered on the waiting list before this policy is implemented. On the date of implementation, approved and pending exceptions will all be ineffective and if your patients do not meet the new policy criteria, you will be able to submit a new exception request. Importantly, your candidates will not lose waiting time during the transition.
Transplant programs should be aware that broader sharing may impact transplant program costs, as it may increase the number, distance, and time of additional heart fly outs and the resources (including donor recovery personnel and transplant program staff) required by your program.
Broader sharing may also impact OPO practices and costs.
Since the proposal requires programs to report cPRA data only if it is available (and does not mandate additional testing), it is unlikely that histocompatibility laboratories will experience increased testing costs.