Guidance for Identifying Risk Factors for Mycobacterium tuberculosis (MTB) During Evaluation of Potential Living Kidney Donors
Summary and Goals
On November 13, 2012, the Board of Directors approved a requirement that all potential living kidney donors undergo evaluation for infection with Mycobacterium tuberculosis (MTB). Targeted rather than universal testing for MTB was felt to be the most efficient way to identify potential donors infected with MTB and decrease the risk of transmission of infection to the recipients. The OPTN Ad Hoc Disease Transmission Advisory Committee (DTAC) was charged with creating a guidance document to assist the transplant community with identifying potential donors at increased risk of infection with MTB. Since this resource is not OPTN policy, it does not carry the monitoring or enforcement implications of policy. It is not an official guideline for clinical practice, and it is not intended to be clinically prescriptive or to define a standard of care. This is a resource provided to members for voluntary use.
MTB Background
Epidemiology and Pathophysiology
Up to 1/3 of the world’s population is infected with MTB; however, infection in the United States is much less common. Reported cases of active tuberculosis have been declining in the United States since 1992 with the majority of cases occurring in foreign born persons. After initial infection with MTB, most people do not develop active tuberculosis; the infection disseminates throughout the body and remains dormant. This condition is called latent tuberculosis infection (LTBI). It is estimated that 4.2% of the U.S. population has latent TB infection. In patients with latent tuberculosis, the highest risk of reactivation and the development of active disease occur in the first year after infection and then declines. Since initial infection does result in live MTB in many organs, however, tuberculosis can be transmitted by other organs used for transplantation from donors who have never had clinical signs or symptoms of active tuberculosis.
FDA-Approved Tests for Latent TB
While active tuberculosis can involve almost any organ and cause many syndromes (e.g., meningitis) a careful medical history and examination will likely identify the majority of patients with active tuberculosis. Latent tuberculosis, by definition, is asymptomatic and specific testing is required to identify patients with latent tuberculosis. The currently FDA approved screening methods for LTBI in the US include the tuberculin skin test (TST) and the interferon gamma release assay (IGRAs): QuantiFERON-TB gold in tube (QFT), and T-SPOT.TB. These tests do not differentiate active from latent TB, and may be negative during times of active infection. The TST test requires injection into the skin and a return visit in 48-72 hours to interpret the test. The IGRAs are blood tests and may show positive, negative, borderline, or indeterminate results.
Indeterminate results indicate that the controls did not work in that patient and this result is more common in immunosuppressed patients. One advantage of the IGRAs is that patients who have had childhood BCG vaccine (very common outside of North America and Western Europe) are less likely to have a false positive test with IGRAs than with the TST test, due to enhanced specificity of the Mycobacterium tuberculosis antigens used in the IGRA assays.
Donor-derived Tuberculosis
As of September, 2012, thirty cases of possible, probable, or proven donor-derived tuberculosis in solid organ transplant (SOT) recipients have been described in the literature or confirmed by DTAC. Four of these were from living kidney donors. Death occurred in 17% of recipients, and disease was often extra-pulmonary and diagnosis was sometimes delayed. Treatment of active tuberculosis in SOT recipients can be difficult, due to multiple toxicities and drug interactions (particularly the profound reduction in calcineurin inhibitor levels caused by rifamycins).
Risk Factors for Active or Latent TB
Since latent infection with MTB precedes the development of active disease, similar risk factors would be expected to be present. One difference is that since the rate of reactivation decreases with time from infection, donors with distant infection would still be at risk for transmission but at a lower risk for active disease. The most powerful easily identifiable risk factor is place of birth with estimated risk of latent TB of 18.7% among foreign born persons in the U.S. as compared to 1.8% among U.S. born persons. Higher risks countries include the first four columns in table one. Accordingly, foreign born persons from endemic regions are already in a high risk category.
For individuals from low risk regions, including the United States, the following factors would be expected to increase the risk of infection with MTB:
RISK FACTORS FOR TB IN INDIVIDUALS FROM LOW RISK REGIONS (INCLUDING THE U.S.)
-
Close contacts of persons with infectious TB
-
Those who spend significant time in areas of the world with high rates of TB (green shaded area on Table 1, and all but the lightest shaded area of Figure 1)
-
>3 months
-
Relief work in a country with high TB risk
-
-
History of injection drug use
-
Persons who reside (or ever resided) or worked in institutional settings which resulted in increased risk of exposure to TB (hospitals, nursing homes, correctional facilities, other health care settings, homeless shelters)
-
National Health and Nutrition Examination Survey (NHANES) data indicate other higher risk groups among U.S. born persons to include:
-
Non-Hispanic black/African American (5.7%)
-
Older age (4.8% >65 years old)
-
Mexican/Mexican American (2.5%)
-
Low income/poverty (2.8%)
-
-
Radiographic evidence of prior tuberculosis on chest radiograph
Donors with a History of Active Tuberculosis or Latent Tuberculosis
In all donors, a history of LTBI or active tuberculosis should be obtained. While limited data is available, some general recommendations regarding donors with a history of MTB can be made. Donors with any question of active MTB should be excluded from donation until further work up and treatment can be performed. Table 2 provides some suggestions for management of potential donors with a history of LTBI, newly discovered LTBI, or a history of active MTB.
Summary
MTB can be transmitted from asymptomatic living kidney donors with LTBI to recipients resulting in significant morbidity and mortality in the recipient. Centers that elect to do targeted testing for LTBI rather than universal testing should focus on foreign-born donors (first four columns of table 1) and U.S. born persons with the risk factors described above. Suggestions for the management of potential donors with latent or a history of active MTB infection are provided in table 2.
Table 1. Tuberculosis Incidence by Country of Origin (WHO 2010).
| >300/100,000 | 100-299/100,000 | 50-99/100,000 | 25-49,000 | <25/100,000 |
|---|---|---|---|---|
|
Africa with exceptions noted Cambodia |
Angola Tanzania Somalia India Pakistan SE Asia with exceptions noted S. Pacific Russia Mongolia North Korea Belarus Uzbekistan Turkmenistan Czech Republic Afghanistan |
China South Korea Lithuania Latvia Iraq Yemen Morocco Algeria Ecuador Peru Bolivia Guyana Suriname |
Spain Portugal Estonia Poland Saudi Arabia Turkey South America with exceptions noted |
North America Australia Europe with exceptions noted Japan Libya Egypt |
Figure 1. World Health Organization Global TB incidence, 2010
(https://www.who.int/teams/global-tuberculosis-programme/tb-reports). Downloaded August 16, 2012.
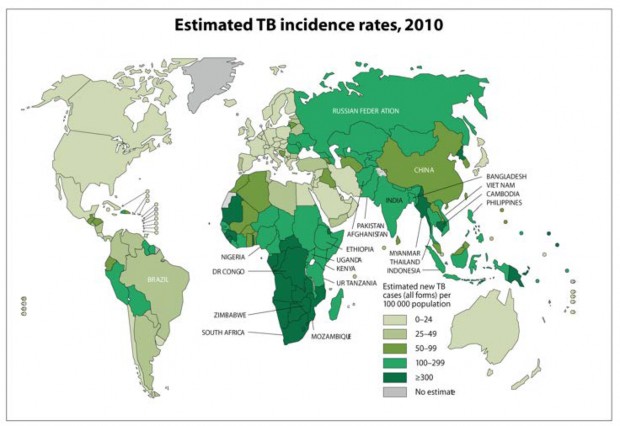
TABLE 2: Management of latent or history of active TB in living donors
HISTORY OF ACTIVE MTB
| Clinical Scenario: Living Donor | Risk for Transmission | Recommendation |
|---|---|---|
|
LATENT TB |
||
|
History of latent TB-treated appropriately |
Lower |
Monitor recipient clinically |
|
History of latent TB-treated insufficiently or not treated or treatment details not clear OR new diagnosis of latent TB-positive TST or Interferon gamma release assay found during pre-transplant evaluation; evaluation finds no evidence of active TB |
Moderate |
Consider deferring transplant if possible until donor has taken some/all of chemoprophylaxis and consider chemoprophylaxis of recipient; monitor clinically |
|
Unexplained pulmonary apical fibrosis in donor without cavitation and without additional testing |
Variable |
Defer donation pending further evaluation |
|
History of active MTB treated appropriately over 2 years ago |
Lower to moderate |
Monitor recipient clinically; consider cultures of previous TB sites if possible. Consider TB prophylaxis of recipient. |
|
History of active TB-site remote from transplant treated appropriately within 2 years. |
Lower to moderate |
Monitor recipient clinically; consider cultures of previous TB sites if possible. Suggest chemoprophylaxis of recipient. |
|
History of active TB-site remote from transplant treated insufficiently and/or with other than standard regimen Excluding disseminated or CNS TB. |
Higher Increased risk if less than 2 years since active TB diagnosis. |
Defer live donors until adequately treated; consider consult with infectious disease specialist; recommend cultures of previous TB sites prior to transplant if possible |
|
History of active renal TB treated appropriately. (If not treated appropriately donation should be deferred until after appropriate treatment) |
Moderate |
Verify treatment; monitor clinically; recommend chemoprophylaxis for recipient; recommend cultures of previous TB site(s); consider consult with infectious disease specialist. |
Adapted from “Diagnosis and Management of Tuberculosis in Transplant Donors: A Donor- Derived Infections Consensus Conference Report”. Morris MI, Daly JS, Blumberg E, Kumar D, Sester M, Schluger N, Kim SH, Schwartz BS, Ison MG, Humar A, Singh N, Michaels M, Orlowski JP, Delmonico F, Pruett T, John GT, Kotton CN. Am J Transplant. 2012 Aug 6.
REFERENCES
Bennett DE, Courval, JM. Prevalence of tuberculosis infection in the Unites States population: the national health and nutrition examination survey, 1999-2000. American Journal of Respiratory and Critical Care Med 2008; 177 (3):348-355 2008
WHO (2010). Global tuberculosis control 2010. Geneva, World Health Organisation: 1-218.
Diagnosis and Management of Tuberculosis in Transplant Donors: A Donor-Derived Infections Consensus Conference Report”. Morris MI, Daly JS, Blumberg E, Kumar D, Sester M, Schluger N, Kim SH, Schwartz BS, Ison MG, Humar A, Singh N, Michaels M, Orlowski JP, Delmonico F, Pruett T, John GT, Kotton CN. Am J Transplant. 2012 Aug 6.
Approved 11/13/2012



