Split Versus Whole Liver Transplantation
December 2016
Caveat: The purpose of this White Paper is to outline potential ethical issues and other considerations relevant to split liver transplantation. The document should not be construed as a recommendation to require that all centers adopt split liver transplantation. Throughout this document, surrogate decision-makers can speak on behalf of patients when those patients lack decision-making capacity.
A disparity exists between the number of donor livers available for transplant and the number of persons on the waiting list for a liver transplant. The practice of splitting deceased donor liver allografts to provide liver transplants for two recipients from one deceased donor could ease this disparity by increasing the number of organs available for transplant.
Since November 2007, an OPTN match run has identified a donor liver as one with the potential to be split if all these criteria are met:
- Donor is less than 40 years old;
- Donor is on a single vasopressor or less;
- Donor transaminases are no greater than three times the normal level;
- Donor body mass index (BMI) is 28 or less.
More than 10% of all deceased donors, and more than 20% of donors less than 35 years old, meet these criteria, yet only less than 1.5% of all donor livers have been split since criteria adoption (Appendix-1). The Ethics Committee (the Committee) supports efforts to increase liver allograft splitting when these (and other) criteria are met, including development of an allocation scheme to optimize use of these donor livers. As described below, the Committee acknowledges that substantial barriers must be overcome before the practice could become more common.
The ethics of optimal allocation
The Committee affirms that optimal allocation policies involving whole liver or split liver allografts should reflect a balance between the principles of equity and utility. Additionally, the Committee notes that the oral principle of respect for autonomy is generally important in organ allocation to the extent that a competent transplant candidate or surrogate retains the right to refuse an organ offer including a split liver organ. The autonomy of transplant candidates (or their surrogates) should not be compromised by situations in which they may feel coerced to make a decision about accepting a particular organ or allowing splitting of a liver to take place. The Committee also recognizes the value of transparency during the process of revising allocation policies so that stakeholders and the public may have adequate opportunity to respond to proposals.
Current liver allocation of deceased donor liver allografts
In current practice, liver allografts are allocated such that priority is generally given to candidates with the highest risk of death on the waiting list. This priority is predicted by the Model for End-stage Liver Disease (MELD), or Pediatric End-stage Liver Disease (PELD) score, and most patients are critically ill before receiving priority for a liver transplant. In addition, liver allocation often prioritizes patients with standardized “exception points” due to hepatocellular carcinoma, hepatopulmonary syndrome, and portopulmonary hypertension, and there is published evidence that non-standardized exception points are not awarded consistently region to region or by race1,2.
The Committee does not believe that current liver allocation is optimal, because it neither takes into account post-transplant outcomes (an important metric of efficiency), nor does it maximize equitable distribution. Proposals to revise the current liver allocation system are under consideration by the OPTN.
In the text below, we consider split liver transplantation in the context of the current allocation system, but also consider how the practice might be implemented under different allocation systems.
Clinical background: The practice of splitting a deceased donor liver allograft
Historically, split liver transplantation has primarily benefited pediatric recipients who are too small to be transplanted with a full-sized liver (Appendix-2). This scenario usually arises when a child is offered a liver that is too large, but the ability to split that liver and transplant the child with the left lateral section (segments II and III; see Figure 1) results in transplantation sooner than if the child had to wait for an age- or size-matched deceased donor liver. Since pediatric donors are less common than adult donors, split liver transplantation has significantly reduced time and mortality for patients on the pediatric waitlist3,4. The “extended right lobe” remnant graft (segments IV, V, VI, VII and VIII; see Figure 1) can then be transplanted into an adult, thus transplanting two candidates from one deceased donor. For pediatric and adult recipients, graft and patient survival after this type of split liver transplantation are similar to those for recipients of whole organs, though complications are more frequent5,6,7,8,9,10,11,12.
Splitting a single liver allograft for two large (e.g., adult sized) recipients is also possible. The two grafts resulting from this type of liver splitting are: “right lobe” (segments V through VIII; see Figure 2), and “left lobe” (segments II, III and IV; see Figure 2). Both recipients in this scenario are usually adults. There is less experience with this type of split transplant and the recipient outcomes are not as consistently favorable13,14,15 though at least one recent report indicates good results may be achieved in low-MELD recipients16.
However, there are a number of clinical limitations to the practice of splitting liver allografts. First, some adult recipients are not well suited to receive a partial liver. Critically ill patients are not optimal candidates for a partial liver because they are less able to tolerate the more-frequent complication(s). A second limitation (noted earlier, Appendix-1) is the limited number of donated livers (more than 10% of all donated livers) that meet OPTN-specified criteria for splitting. A third limitation is center expertise, because split liver transplantation requires substantial clinical experience on the part of both the procuring surgeons and the recipients’ clinical teams. This lack of experience may be one reason why only about 1% of livers are split. In practice, very few centers have consistently performed split liver transplantation over the past decade.
To ground these limitations in concrete examples, we suggest that split liver transplantation may be considered in the following clinical scenarios. These scenarios all assume appropriate center expertise and candidate readiness. Donor “suitability” refers to clinical criteria listed above:
Scenario 1
A suitable donor is identified, a suitable pediatric candidate in whom the left-lateral section is size-appropriate has allocation priority, and a suitable larger (adult or pediatric) candidate is identified to receive the extended right lobe graft remnant (Figure 1).
Scenario 2
A suitable donor is identified, a suitable large pediatric or adult candidate has allocation priority, and a suitable pediatric candidate in whom the left-lateral section is size-appropriate is identified (Figure 1).
Scenario 3
A suitable donor is identified, a suitable candidate (adult or pediatric) too small for the whole liver or extended right lobe graft (but requiring more than the left-lateral section) has allocation priority, and a second suitable adult or pediatric candidate is also identified. In this scenario, the liver is split more evenly (Figure 2).
The ethics of modifying allocation and practice to promote split liver transplantation
The main ethical justification for split liver transplantation is that the practice may increase efficiency when two liver segments can provide greater net survival to two appropriately selected recipients instead of a single recipient. Split liver transplantation could also increase transplant access for pediatric candidates. As discussed in another white paper from the Committee17, children may be considered among the ‘worst off’ transplant candidates, and affording this group some allocation preference is reasonable.
When the index candidate (the candidate at the top of the allocation match run) is a child who requires only the left-lateral section of a suitable donor, we feel it is fair and efficient for this candidate’s center to split the liver for the child and a larger (adult or pediatric) candidate in whom the extended right lobe graft is size-appropriate (Scenario 1; see also Figure 1). Necessary elements of splitting the liver include, but are not limited to, medical suitability of the candidates and donor, as well as current center-specific experience with split liver transplantation. The ethical rationales for this practice are that survival gains from transplantation are provided to two individuals and that the index pediatric candidate is not disadvantaged.
If splitting the donor liver is planned, the transplant center should notify the organ procurement organization (OPO) managing the donor as early as possible, to allow the OPO to offer the remnant graft to other candidates on the match run. According to OPTN policy, if the remnant graft has not been allocated by the start of organ retrieval, the index candidate’s transplant center should offer it to medically appropriate candidates on its list according to their waitlist priority. The goal of these efforts is to ensure fair and efficient remnant graft allocation and minimize remnant graft discard. Ideally, the initial match run for a suitable donor would identify two recipients for consideration before offering the liver as a whole graft: the index pediatric recipient to receive the left-lateral section and a larger (adult or pediatric) recipient for the extended right lobe graft. Notably, since 2001 approximately 85% of candidates have been listed as agreeing to receive a partial liver (Appendix-3). The Committee supports consideration of a change in allocation policy to facilitate this scenario.
Scenario 2 differs only in that the index candidate is not the proposed recipient of the left-lateral section. This results when a larger (pediatric or adult) recipient is the index candidate for a liver from which the extended right lobe graft would suffice, leaving the left-lateral section for a medically suitable smaller pediatric candidate (Figure 1). Again, when a suitable donor is identified, it would be appropriate for the match run to identify two potential recipients: the index adult or large pediatric candidate for the extended right lobe graft and a smaller pediatric candidate for the left-lateral section. This would encourage centers with an opportunity to split a liver to allow an additional child to receive a transplant to do so – again, transplanting two recipients from a single donor. Among other considerations, the index candidate’s individual situation and current center-specific experience, practice, and outcomes are all important in the decision to perform split liver transplantation in this scenario. It is again recognized that critically ill patients may not be optimal candidates for a partial liver allograft and could, therefore, be disadvantaged if all donor livers meeting split criteria were primarily offered only as a split. This disadvantage could be mitigated by allowing candidates with a certain MELD threshold (e.g., >35) to receive priority for the whole liver over candidates who appear on the split liver match run.
Alternatively, and less commonly, the index candidate is an adult or pediatric recipient in whom a right lobe graft (segments V, VI, VII, VIII) or left lobe graft (segments II, III, and IV) would be size-appropriate. As previously mentioned, reported results13-15 from this type of split (Scenario 3) are less consistently favorable than Scenario 1 or 2. Thus, in this case, providing a partial liver to the index candidate and offering the other liver segment to another suitable candidate on the waiting list may not promote efficiency versus whole liver allocation. Given this problem and graft weight/body weight ratio requirements, particularly for high-MELD patients, we do not currently advocate a change in policy to facilitate this scenario.
Informed consent
The principle of autonomy requires that transplant candidates (or their surrogates) have transparent discussion and disclosure of information about allograft quality, expected outcomes with the transplant versus remaining wait-listed, and center experience. These requirements of informed consent should encompass discussion about split liver transplantation where clinically appropriate. These processes of informed consent should first be addressed while the patient is on the waiting list (ideally at the time of listing) and when organs are offered. Patients (or surrogates) may change their decisions about willingness to accept certain types of organs because of changes in clinical status or other reasons. If a center and/or patient decline a split liver offer, they should retain their position on the list; in this scenario, the split liver can be offered to the next candidate on the match run willing to receive a partial liver graft.
The specific clinical circumstances of liver transplantation include challenges to informed consent that the Committee acknowledges. First, liver transplant candidates often become critically ill on the waiting list, impairing their capacity and requiring consent from surrogates. Ideally, informed consent would involve discussions when candidates are capable of understanding the risks and benefits, although candidates are sometimes added to the waiting list when hepatic encephalopathy precludes such discussions.
Second, because a patient’s or their center’s circumstances might change while the patient is on the waiting list, recurring informed consent discussions regarding this issue, along with potential updating of their willingness with the OPTN, are appropriate. Third, transplant candidates or their surrogates should not be put in a position of undue pressure, in which the transplant staff may be perceived as coercing them to accept a split liver transplant at the time of organ offer. Instead, allocation procedures should be developed such that when two patients are identified on the match run that are likely to have favorable outcomes with a split liver, then split liver transplantation should be offered to the candidates as the only transplantation option with that organ, rather than asking the candidates whether they want the entire liver or a split liver. This approach avoids candidate (or surrogate) coercion and recognizes that deceased donor livers – whether transplanted as whole or split organs – are a community resource that should be allocated according to the principles articulated earlier in this document.
Center expertise
With the preceding discussion, the Committee acknowledges that the risk-benefit ratio of split liver transplantation will vary between transplant programs and the acuity of illness of their listed candidates. Our intent is not to require a change in practice if liver splitting is not appropriate for a center; rather, centers with appropriate experience and/or expertise are encouraged to consider split liver transplantation in order to safely increase the number of liver transplants performed. Because of similar outcomes with Scenarios 1 and 2 when compared with whole liver transplantation, changes in allocation and practice (e.g., offering the liver to two recipients as a split) may be appropriate at this time to encourage this type of split liver transplantation, as net survival would be expected to increase. If good outcomes become consistently documented with Scenario 3, changes to encourage this practice should also be considered.
In addition to the individual medical suitability of donors and recipients, the Committee recognizes other stakeholders and/or factors, some with competing interests. If in situ splitting is planned, for example, the donor hospital’s capabilities and resources (personnel, operating room time, equipment) need to be considered. Increased intraoperative time would impact the extrahepatic organ retrieval team(s); the donor may become hemodynamically compromised, impacting all organs and those targeted candidates; if split liver candidates are at different centers, both centers might want their surgeon(s) involved in the splitting, further complicating case coordination. If ex vivo splitting is planned and the candidates are at different centers, additional challenges may emerge. Lastly, intraoperative findings (e.g., anomalous vascular anatomy) may preclude safe splitting. For logistical reasons, therefore, it is likely unfeasible to split every donor liver that meets split liver transplantation criteria. It is worth noting again, however, that split liver transplantation is probably underutilized in contemporary practice.
Each liver transplant program should develop a written protocol that addresses their policies related to split liver transplantation, including information about processes of informed consent. This informed consent should incorporate information about the national and the center’s experience and outcomes with liver splitting.
Additional Challenges
Expansion of the practice of liver splitting poses other challenges. One challenge in the context of current allocation is that MELD-driven allocation usually directs liver allografts toward individuals with the highest level of illness and probability of death on the waiting list, yet split liver allografts may not be well suited to those individuals. Potential solutions might address this challenge. First, under current allocation, many transplant candidates receive exception points due to hepatocellular carcinoma and have MELD exception points despite not being critically ill. Split liver grafts could be directed to these individuals, and/or, as previously mentioned, a minimum MELD/PELD score could be set (e.g., 35) beyond which a liver meeting split criteria is offered to those candidates as a whole graft before allowing consideration of splitting. Second, future revisions to liver allocation processes that take into account post-transplant survival (in addition to waiting list survival) might result in highest priority for individuals who are not necessarily critically ill and might also benefit from split liver transplantation.
A change in allocation policy to identify two individuals on a match run would also have to indicate which candidate’s center would have priority in deciding splitting technique and how the blood supply and bile duct would be shared. It would also need to address whole graft allocation when plans for split liver transplantation are aborted late in the process.
We also acknowledge the possibility that gains in efficiency from liver splitting might come at the cost of a small reduction in access to liver transplantation for persons who are not well suited for receiving a split liver allograft. For instance, individuals with a very large body mass index might not have good outcomes with a split allograft because of organ/recipient size mismatch and might decline these splits. However, the reduction in access to transplantation for obese individuals should be modest. First, the percentage of livers eligible for splitting is low. Second, most obese candidates could accept split livers procured as part of Scenario 1 or Scenario 2 because these split livers are relatively substantial in size. Third, these split allografts are high quality because of the donor characteristics, which counterbalance or may outweigh perceived size disadvantages of the split allograft. As noted earlier, split liver transplantation might also disadvantage high MELD individuals, a problem that could be mitigated if allocation policy discouraged splitting when a high MELD candidate (e.g., MELD >35) had allocation priority.
We also acknowledge that the transplant field has only general information about appropriate recipient candidacy for split liver transplantation. The field would benefit from greater research in this area.
Summary
There are many ethical and logistical issues to be considered in splitting a donor liver. The following points deserve emphasis:
- The transplant community has an ethical obligation to maximize the outcomes from donated organs, while also promoting equity. Split liver transplantation might improve net survival of liver transplant candidates, while also increasing the number of individuals who benefit from transplantation, especially children. Splitting suitable livers for suitable patients is appropriate in centers with adequate experience and outcomes.
- An overriding responsibility of transplant professionals is to properly inform candidates of national and center-specific practices and outcomes of split versus whole liver transplantation. Patients have the right to decide which risks to accept and the right to refuse an organ, including a split liver.
- Informed consent discussions regarding split liver transplantation should take place early: when candidates are initially listed, or, for those patients already listed, when a split liver program is initiated. Recurring discussions will often be necessary, as candidates’ and programs’ circumstances may change.
- The decision to split a donor liver must be made and communicated as early as possible to allow efficient and fair allocation of the remnant graft. Allocation of both resulting grafts on the initial match run for a suitable donor would be optimal. Contingency whole graft allocation is also important, if plans to split a liver are aborted.
- Split liver transplantation involves many stakeholders and complicated logistics. Each stakeholder bears an inherent responsibility to promote appropriate stewardship of donor livers to optimize transplant outcomes and should collaboratively work through logistical issues on behalf of all transplant candidates.
The Committee supports all reasonable efforts to increase the number of transplants safely performed. Changes in allocation and practice to encourage split liver transplantation are a potential means to that end.
Figure 1.
“Extended right lobe graft” in the text refers to segments, IV, V, VI, VII and VIII; “left-lateral section” refers to segments II and III. The smaller recipient would receive the left-lateral section, whereas the larger recipient would receive the extended right lobe graft.
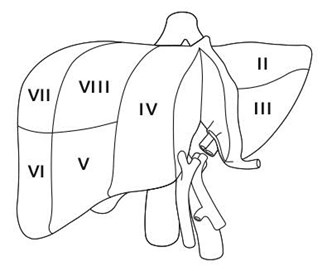
Figure 2.
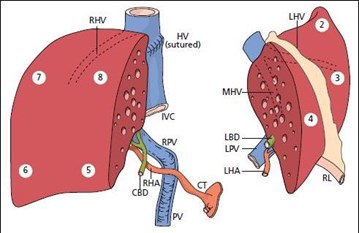
Fig. 2, Springer Science & Business Media.
Appendices (Data generated by OPTN analysts)
1) Of 113,394 deceased donor livers transplanted from 1/1/1995 - 12/31/2015, 1546 (1.36%) were split 259 liver transplants. The criteria listed were adopted in November 2007; from 19 months pre-adoption through 19 months post-adoption (April 2006 - June 2009), 2247 (10.3%) of 21,832 deceased donors met these criteria; during this same period, 218 (1.1%) of 19,644 livers transplanted were split liver transplants.
2) Of 1546 donor livers split from 1/1/1995 - 12/31/2015, 1439 (93%) provided a liver allograft to a pediatric recipient. 6.8% of whole liver transplants were done in pediatric recipients. Additionally, from April 2006 - June 2009, a pediatric recipient was the primary candidate on the match run for 201 (92%) of 218 split liver transplants.
3) From 6/1/2001 - 12/31/2015, there were 151,250 adult registrations for liver transplantation; at listing, 129,276 (85%) noted a willingness to accept a split liver. In 2015, 10,100 (90%) of 11,256 registrants noted a willingness to accept a split liver.
References
- Goldberg DS, Makar G, Bittermann T, French B. Center variation in the use of nonstandardized model for end-stage liver disease exception points. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2013; 19(12):1330-1342.
- Hsu EK, Shaffer M, Bradford M, Mayer-Hamblett N, Horslen S. Heterogeneity and disparities in the use of exception scores in pediatric liver allocation. Am J Transplant. 2015; 15(2):436-444.
- Gridelli B, Spada M, Petz W, et al. Split-liver transplantation eliminates the need for living-donor liver transplantation in children with end-stage cholestatic liver disease. Transplantation. 2003; 75(8):1197-1203.
- Cintorino D, Spada M, Gruttadauria S, et al. In situ split liver transplantation for adult and pediatric recipients: an answer to organ shortage. Transplant Proc. 2006; 38(4):1096-1098.
- Hong JC, Yersiz H, Farmer DG, et al. Longterm outcomes for whole and segmental liver grafts in adult and pediatric liver transplant recipients: a 10-year comparative analysis of 2,988 cases. J Am Coll Surg. 2009; 208(5):682-689; discussion 689-691.
- Spada M, Cescon M, Aluffi A, et al. Use of extended right grafts from in situ split livers in adult liver transplantation: a comparison with whole-liver transplants. Transplantation proceedings. 2005; 37(2):1164-1166.
- Broering DC, Topp S, Schaefer U, et al. Split liver transplantation and risk to the adult recipient: analysis using matched pairs. Journal of the American College of Surgeons. 2002; 195(5): 648-657.
- Renz JF, Yersiz H, Reichert PR, et al. Split-liver transplantation: a review. Am J Transplant. 2003; 3(11):1323-1335.
- Takebe A, Schrem H, Ringe B, et al. Extended right liver grafts obtained by an ex situ split can be used safely for primary and secondary transplantation with acceptable biliary morbidity. Liver Transplantation. 2009; 15(7):730-737.
- Mabrouk Mourad M, Liossis C, Kumar S, et al. Vasculobiliary complications following adult right lobe split liver transplantation from the perspective of reconstruction techniques. Liver Transplantation. 2015; 21(1):63-71.
- Cauley RP, Vakili K, Fullington N, et al. Deceased-donor split-liver transplantation in adult recipients: is the learning curve over? J Am Coll Surg. 2013; 217(4):672-684 e671.
- Cauley RP, Vakili K, Potanos K, et al. Deceased donor liver transplantation in infants and small children: are partial grafts riskier.
- Aseni P, De Feo TM, De Carlis L, et al. A prospective policy development to increase split-liver transplantation for 2 adult recipients: results of a 12-year multicenter collaborative study. Annals of surgery. 2014; 259(1):157-165.
- Giacomoni A, Lauterio A, Donadon M, et al. Should we still offer split-liver transplantation for two adult recipients? A retrospective study of our experience. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2008; 14(7):999-1006.
- Wan P, Li Q, Zhang J, Xia Q. Right lobe split liver transplantation versus whole liver transplantation in adult recipients: A systematic review and meta-analysis. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2015; 21(7):928-943.
- Hashimoto K, Quintini C, Aucejo FN, et al. Split liver transplantation using Hemiliver graft in the MELD era: a single center experience in the United States. Am J Transplant. 2014; 14(9):2072-2080.
- OPTN. Pediatric Transplantation and Ethics Committees. Ethical principles of pediatric organ allocation; Ethical Principles of Pediatric Organ Allocation.



