HCC auto approval criteria changes
View commentsProposal Overview
Status: Implemented
Sponsoring Committee: Liver and Intestinal Organ Transplantation
Strategic Goal: Provide equity in access to transplants
Effective date: 12/12/2017
Related policy notice 12/2017 (PDF - 153 K)
Policy notice 1/2017 (PDF - 157 K)
Policy notice 8/2019 (PDF - 495 K)
Board briefing 12/2016 (PDF - 376 K)
UNOS and OPTN member impact summary 12/2016 (PDF - 861 K)
Executive Summary
The current criteria for automatic approval of hepatocellular carcinoma (HCC) exceptions for liver candidates is problematic, in that they apply to patients who may do well without liver transplant, those who have a poor prognosis after transplant, and potentially excludes patients who may benefit from liver transplant. Additionally, it has been widely shown that successful downstaging of HCC in selected patients is associated with excellent post-transplantation outcome. However, language describing the eligibility criteria for candidates suitable for HCC downstaging through local-regional treatment is absent from current OPTN/UNOS policy, yet nearly all regions currently approve patients who present outside of T2 criteria and have undergone downstaging to within T2. This proposal seeks to make a more consistent national policy regarding HCC patients, increase equity in access to transplants and improve waitlisted patient and transplanted recipient outcomes through modifications to the current standardized HCC exception process.
Read the full proposal. (PDF - 389 K)
Feedback requested
Readers were asked to comment on the entire proposal, but the Committee sought feedback from the community on the following:
The Committee discussed whether the required imaging should occur within a specified timeframe after local-regional therapy. Currently, the proposal only requires imaging after local-regional treatment. Should there be a specific interval of time for the required imaging after local-regional therapy and what timeframe would you suggest?
The Committee discussed that there could be variation among centers on their definition of a residual or recurring tumor, which is a criteria that determines eligibility for standard exception in the proposed policy. Committee is seeking feedback on the community’s definition of a residual or recurring tumor to potentially further clarify the proposed policy.
The Committee also asked whether guidance is needed for review board members to assess any clinical scenarios not meeting criteria in the proposed policy.
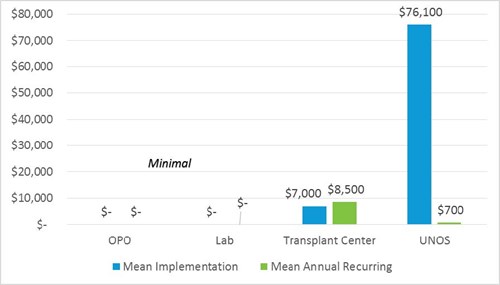
UNOS and OPTN member impact summary
Project size/complexity
UNOS
Major implementation hours include 920 IT hours and 30 member quality hours to update templates and forms, perform site survey, allocation reviews, and member education.
Ongoing effort is minimal.
Project size = Large
Member
Transplant center
Implementation timeframe for transplant centers is minimal, estimated at two months for most centers. Time may be needed to implement systems to track patients in greater detail. System changes include workflow changes, reports, and scheduling changes.
Existing workflow process, unique to each center, is the variable that most impacts implementation and ongoing financial resources. Additional staff time is needed to ensure patients meet all criteria for downstaging, exceptions, or listing. Small centers with fewer staff members may be most burdened, while larger centers may be able to more easily add additional staff hours and absorb financial impact.
Transplant center methodology notes
- One-time implementation cost impact estimate (for most transplant centers) of $7,000 is based on 100 hours of staff implementation time at a blended rate of $70/per hour and includes medical and administrative staff.
- Recurring annual additional budget cost post-implementation is estimated at $4,000-$13,000 and based on average of $50/per hour (RN/MA only) rate at 3-5 additional staff work hours per week for 52 weeks per year.
- There is no cost savings.
OPOs and labs
OPOs and labs are not affected.
Summary of Changes
Policy 9.3.F: Candidates with Hepatocellular Carcinoma (HCC)
This proposal contains two primary policy changes:
- Candidates with lesions meeting T2 criteria but with an AFP greater than 1000 are not initially eligible for a standardized MELD exception. If these lesions fall below 500 after local-regional therapy, the candidate is eligible for a standardized MELD exception. Candidates with an AFP level greater than or equal to 500 at any time following local-regional therapy will be referred to the review board.
- The policy addition describes the eligibility criteria for being included in the downstaging protocol. Candidates meeting the criteria will be eligible for automatic priority after they’ve had local-regional treatment, and if their residual lesions fall within T2 criteria.
The transplant program will be required to submit an updated exception request at the time of extension indicating that their candidate still meets the initial eligibility criteria. This ensures that at the time of extension, their candidate continues to meet the criteria that initially qualified them for MELD exception points.
Current policy contains recommendations on the imaging characteristics used for CT scans and MRIs performed for a HCC MELD or PELD score exception. We will remove those recommendations from policy and add them to the forthcoming HCC guidance document.
What members need to do
Transplant hospitals need to be aware of the new criteria for automatic approval of HCC exception requests.