An important message from the MPSC on best practices for organ verification and other recommendations
Published on: Thursday, September 19, 2024
For the benefit of the transplant community, the Organ Procurement and Transplantation Network (OPTN) Board has authorized the Membership and Professional Standards Committee (MPSC) to disseminate learnings and effective practices observed during MPSC meetings and interactions with OPTN members. Updates will be provided by the MPSC after each of their in-person meetings.
The MPSC recently communicated to a large segment of OPTN members about several recommendations and opportunities for improvement in processes and protocols related to organ recovery and transplant:
- Best practices for organ verification
- Transplant program performance monitoring
- Network security requirements
- Reporting of third-party vendor issues and medical device malfunction
The MPSC is also currently working on several initiatives which include:
- Changes to transplant program performance monitoring criteria for post-transplant outcome metrics
- OPO performance monitoring and the HRSA data collection directive
- Ongoing policy compliance, patient safety, and performance reviews
- Monitoring completion and comprehensiveness of eGFR wait time modifications
These are areas of focus where the MPSC believes they can provide valuable information and tools to OPTN members for support and oversight.
The MPSC is an operating committee of the OPTN Board of Directors. In addition to monitoring for compliance with OPTN Final Rule, policies, and bylaws, the committee supports members through peer review and sharing of effective practices. Find the MPSC’s community updates and other materials on the MPSC resources page.
Best practices for organ verification
We reviewed many examples of safety events that could have been prevented by appropriately completing organ verification processes during procurement and prior to transplantation. Ways to ensure accuracy include:
- Verifying unique identifiers
- Ensuring organ paperwork matches paperwork on the pump lid
- Documenting laterality
- Double-checking internal and external labels
Confirming the organ’s appearance and quality is consistent with what is expected
Members are encouraged to review OPTN policy requirements, with specific attention to OPTN Policy 18.5: Reporting of Patient Safety Events, and utilize MPSC resources to avoid verification failures.
As more teams and contracted services become involved in the organ procurement and transplant process, and as we collectively continue to increase the numbers of donors and recipients, it is imperative to communicate plans clearly and not make assumptions. Everyone is responsible for ensuring the accuracy of the organ – if something seems incorrect, communicate with your team swiftly.
Transplant program performance monitoring
The final phase of the Enhance Transplant Program Performance Monitoring System bylaw change was completed with the implementation of the pre-transplant mortality metric on July 25, 2024. Resources on all four new metrics in the performance monitoring system are available in a toolkit on the OPTN website, including a recording of a webinar hosted earlier this year which focuses on the pre-transplant mortality metric. All four new metrics are risk-adjusted, meaning their calculation adjusts for each program’s respective risk level by taking into account many factors such as program-specific characteristics of the transplant candidates, recipients, and donors.
Benefits of inclusion of the pre-transplant mortality metric are outlined in the OPTN Board briefing paper, and include encouraging programs to:
- Examine how to actively manage the waiting list
- Review and seek to improve system issues that are barriers to getting patients transplanted
Following good waiting list management practices at your program not only benefits your program’s patients, but it can also contribute to increasing efficiency across the transplant system, benefiting patients across the country.
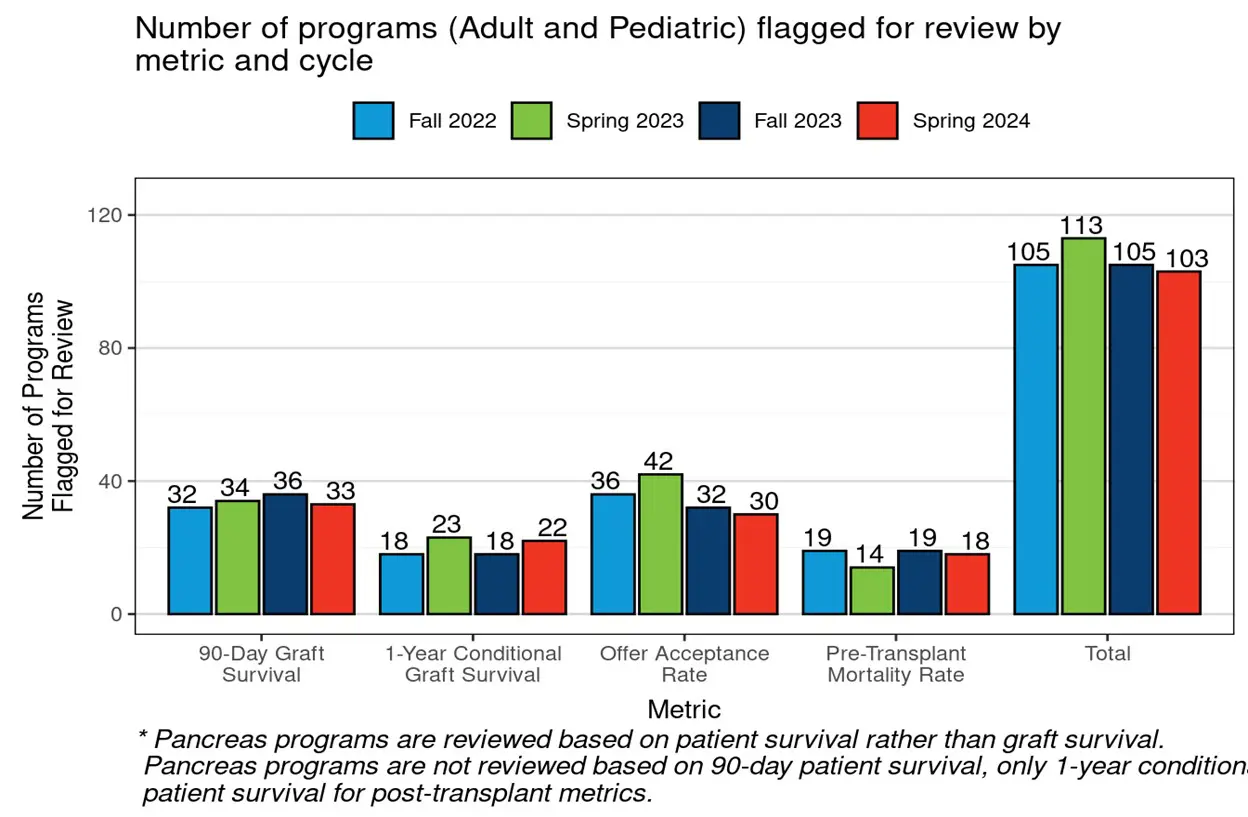
Below is a figure included in the MPSC’s recent review of aggregate data on program flagging under the new metrics. This figure shows the number of programs flagged or that would have been flagged for not yet implemented metrics over the past four review cycles. The 103 programs flagged in July of 2024 represent only 12.7% of total active programs at the time of flagging.
For comparison, an average of 97 programs were flagged for post-transplant outcomes alone during the last two cycles prior to implementation of the new metrics.
Network security requirements
The phased implementation of the Establish Member System Access, Security Framework, and Incident Management and Reporting Requirements policy change sponsored by the OPTN Network Operations Oversight Committee is in process. It is imperative that all OPTN members ensure their ongoing compliance with the new requirements, which include:
- Completion of OPTN training requirements by staff
- Designation of information security contacts
- Completion of an attestation of compliance with information security requirements
Additional information and resources are available in the Member Security toolkit on the OPTN website. Please be aware that noncompliance with the new requirements, such as failure to complete the attestation within the required timeframe, could result in referral to the MPSC for review.
Reporting of third-party vendor issues and medical device malfunction
Vendors such as on-call services, perfusion teams, and organ recovery teams are being increasingly utilized to address the demands of increasing volumes and complexity in our field. While these entities are becoming important partners, not all are currently OPTN members.
OPO and hospital members are encouraged to report issues with third-party vendors and medical devices through the OPTN Patient Safety Reporting Portal that is accessible via the OPTN Computer System. Note that any issues related to medical device malfunction should be reported directly to the Food and Drug Administration (FDA) using the following link, as the OPTN cannot report the event to the FDA on the member’s behalf:
As the procurement and transplantation process grows to involve third parties, it is imperative for the OPTN to monitor what new issues may arise. Having a better understanding of the number, type, and severity of issues occurring will inform how these groups could be held accountable within the OPTN.
Currently, OPTN members are accountable for the actions of their contracted services during the procurement and transplantation process. Members are encouraged to build in after-action quality review and accountability steps into their vendor contracts. If you have best practices you would like to share, please reach out to the OPTN through the contact info below.
For questions or comments regarding this communication, please email MQFeedback@unos.org


