Identifying Risk Factors for West Nile Virus (WNV) During Evaluation of Potential Living Donors
Summary and Goals
On November 12, 2012, the Board of Directors voted to establish policy for the medical evaluation of living kidney donors and to modify existing related policy. This new language includes a requirement to evaluate living donors at risk for having West Nile Virus (WNV). The board also asked the OPTN Ad Hoc Disease Transmission Advisory Committee (DTAC) to create a guidance document that would assist the transplant community with identifying potential donors at increased risk of infection with WNV. This resource is not OPTN policy, so it does not carry the monitoring or enforcement implications of policy. It is not an official guideline for clinical practice, and it is not intended to be clinically prescriptive or to define a standard of care. This is a resource provided to members for voluntary use.
WNV Background
Epidemiology and pathophysiology
WNV is an RNA virus that is transmitted to humans by mosquito bites. Birds are a reservoir for WNV, and humans are a dead-end host for the virus. WNV was first noted in North America in 1999, and human infection has been reported in all states in the continental the United States, the District of Columbia, and Puerto Rico. The number of human infections varies significantly from year to year and from region to region. For example, in 2012 1868 human cases were reported in Texas and only one case in New Hampshire. Furthermore, different counties within a state may experience dramatically different rates of human WNV infection. Even in southern parts of the United States, human WNV infection is seasonal and most transmission occurs during the warmer months when mosquitoes are most active (Figure 1). The majority of infections are asymptomatic; while approximately 20% of infected individuals develop fever and < 1% develop neurological manifestations including encephalitis or myelitis (spinal cord involvement). Thus, donors without any symptoms could transmit WNV to recipients. While various agents have been investigated, no proven treatment for WNV is available.
FDA approved tests for WNV screening and diagnosis
Nucleic acid tests (NAT) and IgM serologic tests are used for screening or diagnosing WNV. NAT testing is currently performed year-round on blood donations in the United States. Because testing the entire blood supply for WNV by individual donation testing is not currently feasible, blood donors are tested year round in “minipools” from multiple donors. When positive minipools are identified by a blood center, individual donors are tested until a period of time has elapsed with no positive WNV results1. There are currently two FDA-licensed NAT donor screening assays; these are routinely performed at blood banks or some reference labs but are not commonly available in hospital laboratories. Serologic tests which rely on the response of the immune system to infection with WNV, particularly the IgM assay performed on cerebrospinal fluid, are commonly used to diagnose neuroinvasive WNV. IgM is detectable for a median of about 5 months after infection and is not used in blood donors to screen for WNV, although it may be performed for use in donor counseling. IgG typically remains positive for life, and would not be useful to routinely screen for WNV.
Donor-derived WNV after solid organ transplantation
No proven cases of donor-derived WNV infection from living donors have been reported. However, 9 deceased donors have transmitted WNV to 18 of 26 (69%) recipients. In at least two cases, the source of the WNV was blood transfusions received by the donor. Disease in recipients typically developed soon after transplantation, and outcomes have been poor with encephalitis or other severe neurological disease commonly occurring. In some cases, transmission has occurred from NAT negative IgM positive donors, suggesting that WNV may be present in organs and transmissible after the virus has cleared from the plasma.
Considerations for WNV screening
Risk factors for WNV exposure
Since the transmission of WNV is limited by geography and season, you should consider certain factors when determining whether or not to perform laboratory testing on a potential living donor. A positive test result obtained during a period of WNV inactivity is more likely to represent a false positive than when the test was performed during periods of higher activity. Any false positive result could result in unnecessary delays to the transplant.
Factors to consider in evaluating donors for possible of WNV infection
- Has human infection with WNV virus been recognized locally this WNV season?
- Has the donor travelled to an area with human WNV activity this WNV season?
- Has the donor ever been diagnosed with WNV fever or WNV neuroinvasive disease?
- Has the donor had an undifferentiated febrile illness within the current WNV season?
- Has the donor had significant mosquito exposure this WNV season?
Geographic and seasonal factors to consider
Suggested screening strategies for WNV in living donors include universal year-round testing of all donors versus targeted testing during periods of human WNV activity. As the yield of testing during the winter months is likely of low yield, and given the potential for IgM to remain positive after resolution of the illness, year-round testing should be discouraged unless programs are unable to manage the complexity of more targeted testing strategies. Targeted testing strategies could include testing during a pre-determined timeframe, or testing donors only during times of human WNV activity in the area where the donor lives or has traveled. A method for identifying human WNV activity would involve communicating with a local blood bank and determining when they have shifted from mini-pool to individual donor testing. Under this strategy, you would test a potential donor only when there is human WNV activity. This would be indicated by blood donors undergoing ID NAT. Alternatively, testing could begin each year in the spring (May) and conclude in the late fall (November) encompassing the months of highest human WNV activity. Given that human WNV rates vary greatly from year to year and even county to county, targeted testing based on real-time measurement of local WNV activity at local blood banks will be the most cost- effective strategy and will reduce the number of false positives. This strategy will, however, be more complex and time-consuming. Some institutions may already be using a local or regional blood bank testing lab to test other material (e.g., peripheral stem cells used in stem cell transplantation). In contrast, the strategy of testing during a defined time period regardless of local WNV activity will be simpler to implement, but both costs and false positive rates would be expected to be higher. Table 1 describes the advantages and disadvantages of different testing strategies.
WNV diagnostic and screening assays
For donors identified to be at increased risk for WNV, testing would optimally occur within 2 weeks of donation (potentially at the same time you would test for blood-borne pathogens). As discussed above, laboratory tests that could be used to screen for WNV include NAT tests and IgM serologic tests. Only NAT testing is licensed by the FDA for screening organ donors, and NAT testing should be included in any screening strategy. The performance of IgM assays for donor screening has not been evaluated and the performance of the assay for this purpose is uncertain. However, it should be noted that in some cases NAT negative donors have transmitted WNV to recipients and adding IgM testing to NAT may increase the sensitivity in identifying such donors. Table 2 describes the characteristics of NAT and IgM testing.
What to do with positive screening tests or a history of WNV disease
A reactive result on a screening NAT test may indicate active WNV infection or may be a false positive result. Patients with active WNV infection are at risk of transmitting WNV via organ donation. Given that up to 10% of blood donors with a reactive NAT fail to be reactive on repeat NAT but are proven to have WNV infection by serological follow-up testing, identification of a false positive NAT is very difficult. The FDA does provide guidance for interpretation of WNV testing in blood donors. For patients with initially reactive NAT testing, the FDA recommends that the blood donor be deferred for 120 days. As the duration of persistence of WNV in organs is unknown, there is insufficient data to determine if these recommendations are appropriate for solid organ donors, although re-evaluation of potential donors and use of an organ with negative repeat NAT testing after 120 days would likely be safe. If there is concern that the initial reactive NAT represents a false positive test, additional testing using the same assay or an alternate NAT of comparable sensitivity in addition to a cleared test for antibodies to WNV may be of value in test interpretation. If any repeat test (either NAT or WNV antibody) is positive, the patient likely has WNV infection. In the case of a reactive NAT that is non-reactive on repeat testing and tests for antibodies to WNV are negative, the biologic significance is unclear. Local expertise should be used in making decisions regarding timing of organ donation and additional testing on initial NAT reactive donors.
Licensed NAT assays are, however, highly specific, therefore the false positive rate of NAT testing is expected to be very low, particularly if testing is only performed when human WNV is active locally (or if recent donor travel resulted in exposure). If testing is performed during periods of low or absent human WNV activity, however, positive results are more likely to be false positives.
Organ donors with a history of WNV fever, CNS disease, or positive screening assay for WNV at the time of blood donation should be deferred until at least 120 days has elapsed from those events. You should perform NAT testing (but not serologic testing) again after 120 days have elapsed, and if negative at that time, the organ donor may be used.
Exposure management
Advise potential living donors to avoid mosquito exposure, or to at least use insect repellent during periods of mosquito activity. Further, a potential living donor should report febrile illnesses to his or her transplant center. If your evaluation suggests the possibility of WNV infection, you should consider testing.
Summary
Current OPTN policy requires that living donors be evaluated for risk of transmitting WNV to recipients. Since the majority of WNV infections are asymptomatic, clinical screening will not effectively identify infected donors. Laboratory screening is thus recommended during periods of human WNV activity where the donor lives, works, or travels. Establishing a relationship with local blood banks allow transplant centers to conduct testing only during periods of human WNV activity and reduce false positive results and as well as testing costs. Seasonal testing during potential periods of WNV activity (May to November) is an alternative strategy but would potentially have a higher false positive rate and be less cost effective. While transmission of WNV from NAT negative (no viremia) but IgM positive donors has occurred, the performance of IgM assays as screening tests has not been validated and cannot be routinely recommended.
| Year-round testing(1) | Pre-defined WNV season(2) | Local WNV activity(3) | Routine communication with local blood bank required (4) |
|---|---|---|---|
| Ease of implementation | Simple | Simple | |
| Positive predictive value | Lower during periods of human WNV inactivity | Intermediate depending on level of human WNV activity | High |
| Cost effectiveness | Least | Intermediate | Most |
-
This strategy not recommended
-
May to November
-
Testing triggered by switch from mini-pools to individual blood donor testing at local blood bank. Testing stops when WNV activity no longer noted and blood banks switch back to mini-pools.
-
Some centers may already have an arrangement with blood bank laboratories to test other materials (e.g., stem cells)
| Nucleic Acid Tests (NAT)(1) | IgM (serology)(2) | |
|---|---|---|
| Available Tests | Procleix West Nile Virus Assay COBAS TaqScreen West Nile Virus Test | various |
| FDA licensed for organ screening | Yes | No |
| Availability | Blood bank testing labs Reference labs | Reference labs State Health Departments |
| False positive rate | Low | Likely higher than NAT, but not evaluated for donor screening |
| Indicates active infection | Yes | Remains positive for median of 5 months; active infection may have cleared |
| Required for blood donor screening | Yes | No |
-
Should be used as part of any testing strategy
-
Consider in combination with NAT testing but will increase false positive rate
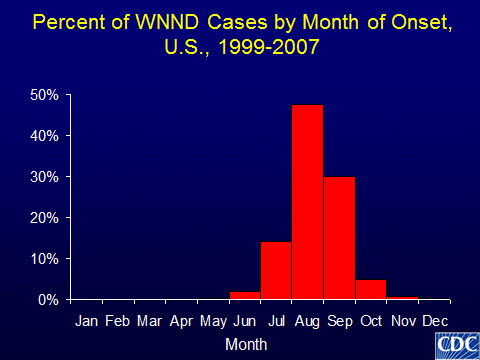
Figure 1: Months of typical WNV activity in the United States
References
Centers for Disease Control, Division of Vector-Borne Diseases, WNV Home Page http://www.cdc.gov/ncidod/dvbid/westnile/index.htm
U.S. Food and Drug Administration. Guidance for Industry: Use of Nucleic Acid Tests to Reduce the Risk of Transmission of West Nile Virus from Donors of Whole Blood and Blood Components Intended for Transfusion http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guid ances/Blood/UCM189464.pdf
1 At this time, there is insufficient data to support recommendation of uniform threshold criteria for switching from MP-NAT screening to ID-NAT screening. Pending development of suitable uniform threshold criteria, FDA considers it appropriate for each blood establishment to define its own threshold criteria for switching from MP-NAT to ID-NAT screening and for reverting to MP-NAT screening.



